(1) Bell, Douglas, et. al. Multi-centre experience with 500 CardioCel® implants used for the repair of congenital heart defects. The Annals of Thoracic Surgery. 2019; S0003-4975(19)30843-4
(2) Neethling, WM, et al. Enhanced biostability and biocompatibility of decellularized bovine pericardium, crosslinked with an ultra-low concentration monomeric aldehyde and treated with ADAPT. The Journal of Heart Valve Disease. 2008; 17:456-463; discussion 464.
(3) Neethling WML, et al. Mitigation of Calcification and Cytotoxicity of a Glutaraldehyde-Preserved Bovine Pericardial Matrix: Improved Biocompatibility After Extended Implantation in the Subcutaneous Rat Model. The Journal of Heart Valve Disease. 2010; 19:778-785.
(4) Brizard et al. (December 2014). New engineering treatment of bovine pericardium confers outstanding resistance to calcification in mitral and pulmonary implantations in a juvenile sheep model. The Journal of Thoracic and Cardiovascular Surgery. Vol 148, No 6, Pgs 3194-3201.
(5) Neethling, WM. Transdifferentiation and remodeling of a tissue-engineered collagen scaffold in the ovine carotid model: an experimental pilot study. Journal of Vascular Surgery. 2017; 65(Supple):195S
(6) AAMI/ISO 11737-2: Sterilization of medical devices—Microbiological methods, Part 2: Tests of sterility performed in the validation of a sterilization process.
(7) Neethling, WM, et al. Evaluation of a tissue-engineered bovine pericardial patch in paediatric patients with congenital cardiac anomalies: initial experience with the ADAPT-treated CardioCel® patch. Interactive CardioVascular and Thoracic Surgery 2013;1–5.
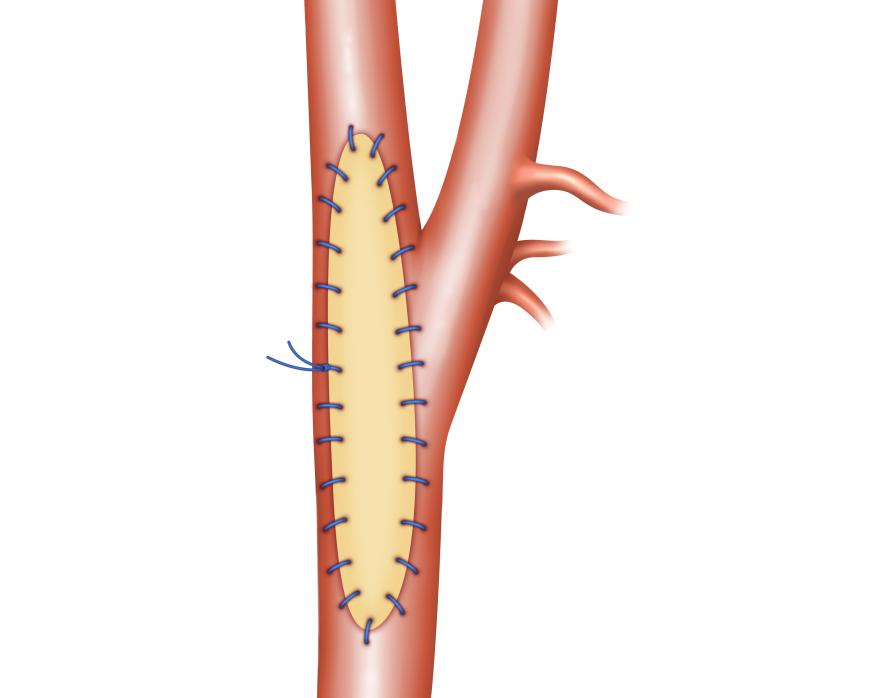
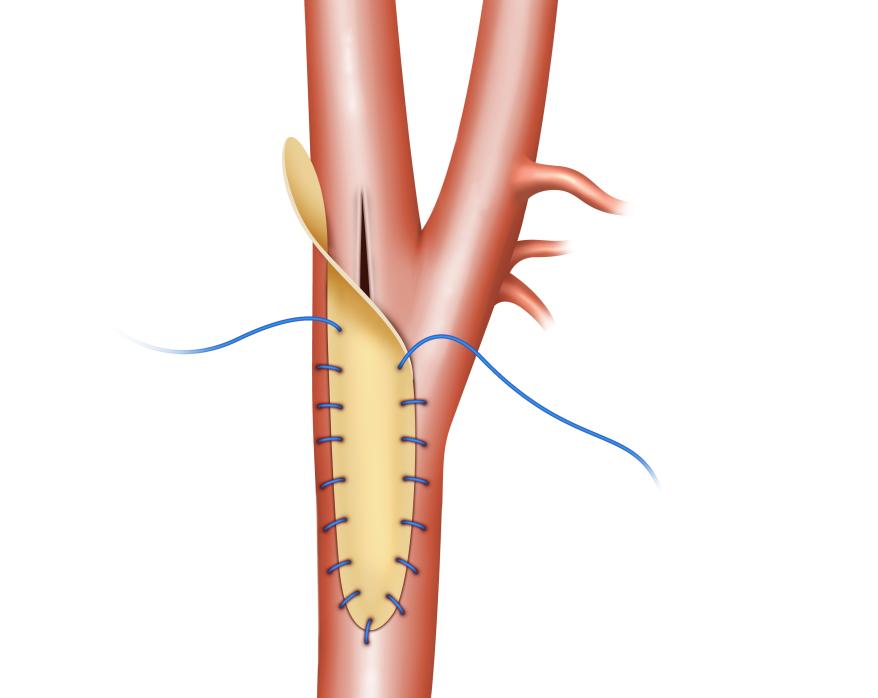
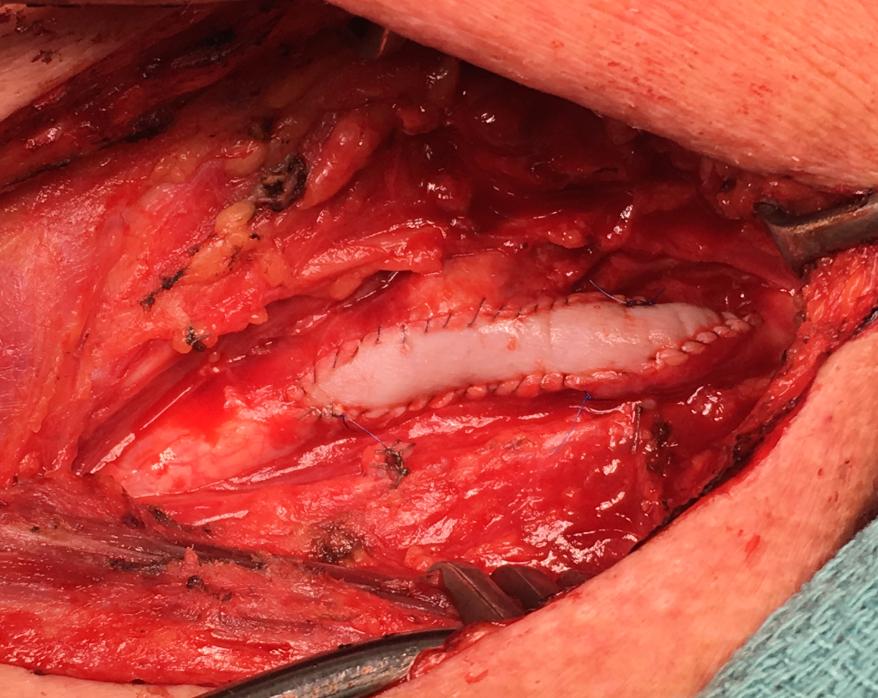
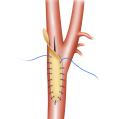
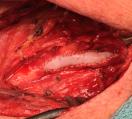
(8) Nelms, Justin, et al Carotid Patch Angioplasty with a novel collagen scaffold following carotid endarterectomy; 2016.
(9) Muto A, MD, PhD, el al. Journal of Vascular Surgery. 2009 July; 50(1):206-213.